Reports of limited effectiveness in mpox cases using the antiviral Tecovirimat SXRG, commercially known as TPOXX, have forced the EMA to urgently review this drug. The review was instated on July 24, 2025, following the latest clinical trial data that have raised doubts about the real-world effectiveness of the drug.
Tecovirimat was first approved in Europe in 2022 under exceptional circumstances, mainly on the basis of animal studies and limited human data. With now several human trials mostly completed, showing conflicting results, regulators are indeed going back to evaluate what the drug has to offer for the treatment of mpox-a viral disease formerly known as monkeypox.
Limited mpox effectiveness of Tecovirimat SXRG (TPOXX) prompts urgent EMA review.
Why has EMA opened the review?
The EMA has acted on the new evidence. Three independent studies produced discouraging results on Tecovirimat’s clinical performance against mpox. These trials—STOMP, PALM007, and UNITY—showed no major differences in symptom resolution as compared to a placebo.
The STOMP trial was a multinational trial that was held in numerous countries, including the US, dealing with the novel strain, clade IIb, currently predominant in global outbreaks. On the other hand, the PALM007 trial, launched in the Democratic Republic of Congo, studied infections caused by clade I. Both concluded that the drug did not reduce the lesion healing time.
With no new major safety risks being uncovered, the EMA questions whether the benefits of Tecovirimat SIGA outweigh the potential risks in light of the lack of proven clinical improvement demonstrated in this set of studies.

EMA responds after three studies show poor clinical results for Tecovirimat in treating mpox.
What exactly did the trials reveal?
In reality, no treatment with SIGA Tecovirimat hastened patient recoveries in trials. Earlier studies of STOMP and PALM007 also did not witness accelerated lesion resolution or improved pain scores.
Doing more to weaken the drug’s case are the conclusions reached in the UNITY trial. Although the sizes of the trials were modest and included patients of varying severity, the consistency in the results raised red flags.
Headache and nausea, each occurring in nearly 10% of the patients, made out the most commonly reported side effects. These are not classified as serious but could, nonetheless, be distracting for patients coming into recovery.
Does this change its current authorisation?
Tecovirimat SIGA has received a treaty-wide authorisation in Europe in January 2022. The authorisation was given under exceptional circumstances, seeing that there are no therapies available for the treatment of orthopoxvirus infections in humans, such as smallpox, cowpox, and mpox. Strong animal data supported the approval, with only limited pharmacokinetic modelling in humans. The EMA has, however, now initiated the process of reassessing whether such exceptional status is still warranted in light of broader real-world data in humans now available.
This, however, is not a suspension nor a recall. The drug retains its availability, but the status of marketing authorisation shall be more deeply assessed. The assessment shall be performed by the Committee for Medicinal Products for Human Use (CHMP), which shall thereafter advise on whether changes to the usage recommendations or product information are warranted.

CHMP to assess Tecovirimat and recommend potential updates to usage or product information.
Who still receives the drug—and why?
Tecovirimat SIGA is still used under special national protocols. As for the United States, the CDC allows under the expanded access program for the medical use of it in some patients, especially those with severe symptoms or underlying conditions such as immunosuppression, HIV, or transplant history.
From hospitalisations or anecdotal data, it appears the drug has been helping improve outcomes in high-risk patients. This includes some examples of shortened duration of hospital admission and lowered viral load. In the absence of trial results that prove it, science-based conclusions cannot be drawn from what are merely observations.
New studies such as PLATINUM-CAN and EPOXI aim to clarify the drug’s effectiveness within these vulnerable subpopulations. Results from these trials are currently pending and may influence the EMA final ruling.
What are the likely outcomes of the EMA’s review?
A review can lead to different outcomes, such as greater restrictions on use, updated prescribing information, and/or safety information. Of course, in the worst-case scenario, the EMA may withdraw the marketing authorisation if it is deemed to have no efficacy.
Experts do not envisage a full withdrawal unless future studies confirm there is no benefit even to high-risk populations. What is more likely would be the shrinkage of indications so that only the most vulnerable groups or emergency-use-type indications would remain.
After a complete analysis, the CHMP will publish the recommendations. Then, the European Commission will decide on whether the changes should be applied across the EU.
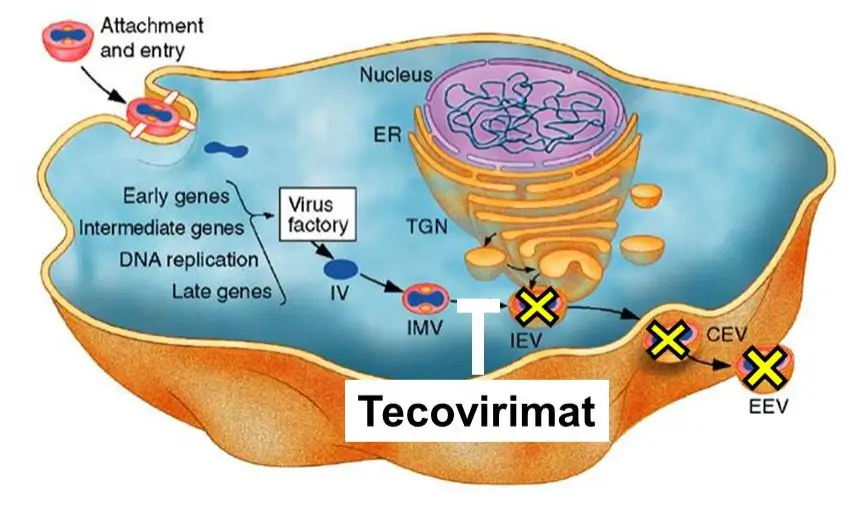
Tecovirimat blocks the formation and release of enveloped virus particles during mpox replication.
What does this mean for Mpox management globally?
In mild to moderate cases, Tecovirimat SIGA retains limited efficacy, yet stands as one of the lone antivirals against mpox. With nothing else circulating in the approved treatments, healthcare professionals are limited to giving supportive care.
Without any intervention of antivirals, most patients recover well; however, severe cases may include the formation of extremely painful skin lesions, prolonged shedding of the virus, and complications arising from secondary bacterial infections. Herein still lie the scenarios where even marginal drug benefits might prove to be clinically significant.
Such outlines make the EMA review all the more significant. The EMA review might have ramifications worth considering as to how global mpox treatment options are proceeding, at this time when outbreaks are still occurring across various continents.
Also Read: Unauthorised AI in Healthcare Sparks Patient Privacy Fears
Final Word: EMA’s Review Signals Turning Point for Mpox Drug
Tecovirimat SIGA was approved under “exceptional circumstances” owing to the rarity of mpox, based only on animal data. Annual reports about the benefit-risk profile of this drug are required as part of the approval. The current post-authorisation review by the EMA, commissioned by the European Commission, intends to reassess the overall safety and effectiveness of the product.
At the heart of this review are the data from the PALM007 trial, which was published in the New England Journal of Medicine and included 597 clade I mpox patients in the Democratic Republic of the Congo. It concluded there was no clinically meaningful decrease in duration of lesions: an average of 7 days with Tecovirimat versus 8 days with placebo. Mortality was 1.6%, due mostly to quality hospital care.
Depending on its findings, the EMA could really lay down the clinical frame of how Tecovirimat SIGA is to be used in Europe and possibly in the treatment of mpox elsewhere. In this way, the outcome may direct subsequent regulatory and clinical pathways in the worldwide management of this disease.
Crafmin.com – Real-time news and insights in Crypto, Mining, Tech, AI, Forex, and Global Markets.

