Engineers and medical professionals are embedding machine learning algorithms in the human body. The use of edgeAI is freeing these applications from cloud connectivity because the data will be processed in a zero-latency setting, such as in hearing aids, pacemakers, and neural interfaces, so treatment can be given instantaneously with personalized reactions, thus diminishing complications and follow-up care. It can be seen now that the technology is developing quickly, with new products and articles being introduced rapidly. (MDPI)

How Edge AI Implants Could Transform Healthcare From Inside the Body (Image Source: CloudxLab)
How “On-Device” Intelligence Upends The Rules
Conventional medical devices transmit data for analysis against a cloud service or a hospital’s database. It is efficient for batch analyses but would be useless in split-second decision-making and in maintaining one’s privacy, considering a device with reasoning capabilities can do so.
They operate with dramatically low energy and memory constraints. They need to be understandable, trustworthy, and updatable over many years. When innovators meet these requirements, implant devices progress from passive sensors to active collaborators in care, adapting treatment currents, alerting for emergencies, or providing a ‘micro-dose’ treatment only when and if required. New technologies in cochlear and cardiac applications represent the transition from data-collecting devices to decision-makers.
Applications Of Sonochemistry And Sonophysics: From Hearing To The Heart
This year, cochlear implant manufacturers introduced devices capable of executing learning algorithms within the implant itself. The devices allow sound processing tailored to a specific user’s needs and accepting updates for better performance with passage of time. This means users’ devices can adjust according to the changing hearing environment without a visit to the medical centre.
Cardiology is closely followed, too. Researchers develop pacemaker and ECG wearable prototypes with capabilities to preprocess, categorize, and even forecast some types of Arrhythmias on the device itself, decreasing latency for priority warnings and the quantity of data needing remote analysis. Even self-sustaining designs tap physiological movement for their power source, introducing possibilities for long-lasting devices that think independently from substantial batteries.
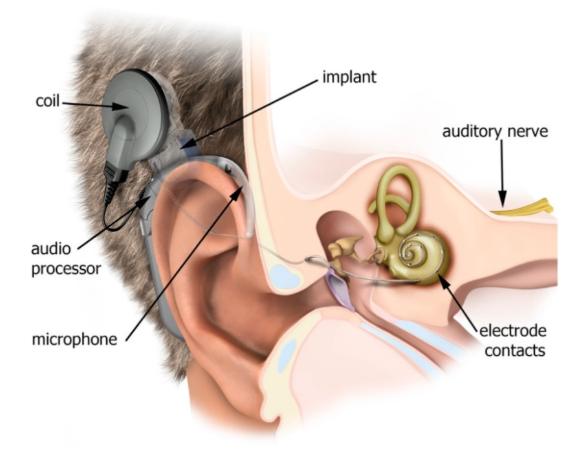
Implants now adapt and analyse on-device, personalising care instantly. (Image Source: MDPI)
The Technological Tightrope: Power, Safety, And Upgradability
The models for devices are much smaller than those used for cloud computing solutions. Technologists favor straightforward, understandable algorithms, such as decision trees, reduced classifiers, and greatly stripped-down neural networks, because these models can be run reliably on microcontrollers.
Energy is the strict resource constraint. Implants must live for years, with surgery required for replacements. Engineers use hardware/firmware/model co-design and architecture search tools to maximize these lifetimes and extend them via over-the-air updates so models can be improved upon without a surgical follow-up visit, although these update paths need to be secure and auditable.
The latest literature and industry products reflect these broad approaches mentioned above.
Clinical Value: Rapid Action, Reduced Follow-Ups, Tailored Treatment
How Is This Relevant For Today’s Patients?
- Immediate answers: A device can anticipate and prevent a life-threatening heart condition, and definitely warn the medical professional and victim in an instant, rather than after cloud computing is finished.
- Tailored therapy: Devices learn what works for the person and adjust therapy based on individual needs. That’s therapy on demand, rather than according to the schedule of a clinic.
- Less friction: Remote monitoring is smarter; only significant events trigger notifications for healthcare professionals.
- It is not a distant promise: Reports from clinical trials and the launch of devices in 2025 indicate the initial victory aimed at chronic diseases requiring continuous adjustment.
Privacy/Data: Where A Strong Patient Role Is Beneficial
Edge implant technology moves sensitive processing from the cloud to the body itself. This will reduce the amount of data being passed and will enable users to control what is being outputted from their devices directly. This is an improvement in terms of users’ privacy because previously, sensitive information such as biometrics would be streamed directly to a third party continually.
However, device-based processing includes other considerations, such as who is able to update the model and what records exist for proof of the device functioning within a safe manner. Regulating entities and device manufacturers are developing these audit paths and secure update capabilities for these devices.
Regulatory Assessments
The rapid advancement of medical devices with machine learning capabilities has prompted regulatory assessments concerning these new technologies and devices.
The Regulator’s Perspective
The regulator’s concept of caution
Medical regulatory bodies are already alert about the dangers and potential of these devices. It is a new era of greater emphasis on evidence, explanatoriness, and post-market surveillance, with a new level of performance criteria for long-term use in the body, often for decades.
It doesn’t inhibit innovation; rather, it drives it. Today, device makers design solutions with auditability and updatable lifetimes, built a mind shift for devices used by consumers. Today’s regulatory environments in key market segments have hardened in 2025, forcing industry players to show the value of on-device learning with acceptable risk.
Engineering Challenges And The Way Ahead
There exist some tough technical issues remaining between prototypes and widespread acceptance:
Harvesting Power And Energy Efficiency
Advances in energy sources and efficient inference engines will increase implant longevity. Development projects for energy-harvesting ECG monitors offer a glimmer of what may be achievable.
Scaling Model Validation
Tiny models will require strong clinical validation so they won’t miss serious adverse events that occur rarely. This will need new types of clinical trials and longer follow-up periods.
Interoperability And Standards
If medical devices implant the possibility of clinical data, they must integrate with medical records and hospital routines with ease. Standards always need improvement, but in a hurry.
There Is Progress Being Made
The academic reviews and standards proposals available in late 2024 and 2025 reflect a movement towards a mature stage in materials, firmware, and clinical evidence.
The Human Side
What Patients And Clinicians Say
Patients always talk about outcomes, better hearing, regular heart rates, fewer seizures, ease of use reduced daily burden. Clinicians appreciate solutions that filter out genuine needs, relieving them from information overload. Testimonials from first followers include fewer calls to the clinic, rapid recovery from seizures, and devices designed for real life, not a laboratory setting.
Technology alone will not establish trust. There will always be a need for communication, education, and medical supervision. The most effective implementations will integrate device-level intelligence with intuitive control and transparent tracking so users can see what their device is doing and why.

Patients want results, clinicians want clarity; smart implants build trust. (Image Source: Auditdata)
A Summary Of Startups’ Rights
The market breaks up into a few bets:
Component Innovation
- Ultra-low power chips, biocompatible sensors, and energy harvesters.
Verified Models
- Smaller algorithms are designed with explainability and safety in mind.
Secure Update Eco Systems
- Systems where devices can evolve without additional risks.
Condition-Specific Solutions
- Condition-specific solutions for hearing, cardiac, neurological, and metabolic implant devices in which continuous adaptation is cost-effective.
- Early leaders who get safety, longevity, and physician workflows right will have a strong market advantage.
Deep Case Study
The Cochlear
Hearing technology illustrates the value of on-device intelligence in providing user wins.
Contemporary cochlear technology is a hybrid solution involving both an external device and an implantable array of electrodes. The implant uses personalized maps and local classifiers, capable of handling noisy conditions. Patients see fewer physician visits and clearer speech in a noisy setting. From a clinical standpoint, these outcomes represent increased device usage and performance in communications and a better quality-of-life factor. The new device activity and reviews reflect the reality of an imminent market within this area of technology.
Realistic Case Study Example: Heart Devices With Triaging Capabilities
Currently, cardiac devices already use continuous monitoring; adding decision logic alters outcomes. Devices that can preclassify the types of arrhythmias cut false alarms and reaction times. Auto-powered prototypes promise solutions for devices lasting for decades without replacements. Tests have favoured preliminary diagnostics and warnings with fewer transitions, necessary for both CHF and arrhythmias. It will take a coordinated launch in specific facilities before mass use can be expected.
NEWS: Neuralink has released what their product evolution will look like in the next few years.
Highlights:
• Q3 2025: They are planning to implant directly into the speech cortex to directly decode attentive words from brain signals to speech.
• 2026: Triple number of… pic.twitter.com/GMke0CbCNE— Sawyer Merritt (@SawyerMerritt) June 27, 2025
Inside The Device: Miniaturized Architecture And It Works
Consider an implant to be a small, fully functional device with its control circuit minimized: sensor → processor → actuator/wireless → energy source. The key is developing decision-making functionality within this circuit so the device can function without cloud assistance.
Designers use three levels of work breakdown. First, Signal Conditioning is a process involving the compression of bio-signals, including ECG, spikes, and sound. Second, Edge Inference uses rule-based systems in order to make a decision about a treatment change. Third, Control and Communications involve a treatment change or an abbreviated log of an event.
It is hardware design choices that impact design considerations in ML inference for IoT devices. Very-low-power inference microcontrollers, custom-designed neural processors, and secure processors for crypto verification have surfaced in design work. There appear to be architectures available in the research and industry community designed around packing useful ML models into a small memory footprint of kilobytes with a consumption level measured in microwatts.
Energy: Batteries Are Not The Only Solution
Replacing the battery is equivalent to surgery. This situation forces the need for creative energy solutions.
For shorter-term devices, high-efficiency batteries and low-power chips remain in use. Long-term implant devices exploit energy harvesting from heartbeat, movement, and even temperature differentials. New technical analyses and publication articles describe self-powered ECG devices and triboelectric energy devices with potential uses in medical implant devices. Although these ideas don’t answer all the issues, they change the cost trajectory of products with longer lifetimes and fewer and less costly replacements.
Security And Updates: Patching Without A Scalpel
It is necessary for intelligence built into devices to evolve with time, but updates should avoid generating new risks.
There is a shift among manufacturers towards signed and auditable update pipelines. The devices first check firmware/model signatures in secure hardware components before proceeding with any updates. They record who made the update, when it happened, and what updates were made. Regulators increasingly demand these records alongside other data in the premarket submission package. This design meets two requirements simultaneouslyenhancements and protection for the patients.
Regulation By Region: What Vendors Must Prove
Today, the regulators think in a lifecycle paradigm; they are concerned about design, validation, and what ultimately happens post-market with a device.
-
United States (FDA)
Lifecycle management is prominently featured in the most recent guidelines (January 2025 Draft and associated documents). There is a demand for change control plans related to adaptive functionality, human factors evidence, and real-world monitoring plans. There will be strict requirements for the premarket evidence required for those implant devices that automatically change therapy.
-
European Union
The Medical Device Regulation (MDR) rule and the EU Artificial Intelligence Act work together here because many embedded solutions would be classified under ‘high-risk’ areas with stronger requirements for conformity assessments and clinical evidence. Suppliers will be required to include explainability and auditability functionality right from the start.
-
Other Markets
Other markets include Australia, Japan, and Canada, which meet the high-bar international standard. Manufacturers intending to launch products in international markets must design for the toughest market among those in which they intend to market products to prevent costly reworks. There is an expected submission of evidence regarding the performance of a model, with change control strategy submissions being an improvement over past requirements.
Clinical Validation
Different Trials For Different Claims
Implant safety can be demonstrated only with long-term follow-up and clever endpoints.
For diagnostic intent use cases, such as analyzing rhythm disorders, a care developer would be required to meet a need for both sensitivity and specificity with reference to a clinical gold standard. For a closed-loop therapeutic use case, involving a change in the level of stimulation/dose with an implant, clinical benefit and safety would need to be demonstrated in clinical trials with a broad range of users. There will be a need for multi-center clinical trials, follow-up cohort studies, and a detailed record of adverse events. A series of reviews and articles for 2025 dwell on this new paradigm of evidence generation within the life cycle of medical
Ethics, Consent, And The Human Contract
Smarter implants pose philosophical and practical issues of much greater importance than the technology itself.
Patients need to be able to opt into a device that learns with the passage of time. Clinicians need access and a ‘veto’ function for significant changes in behaviour. Patients need transparency about what causes a therapy switch, what data is leaving the device, and opt-in/out access to updates.
Ethicists demand a ‘meaningful human oversight’ involving a ‘partnership’ approach where ‘the device would support, rather than substitute for, the physician-patient relationship.’ This will be ensured by ‘good communication’ and ‘patient-centered decision-making.’
Smart implants require patient consent, transparency, and clinician oversight. (Image Source: MDPI)
Investment Landscape: Where Funds Will Flow Next
There are three types of investment tracked, namely components, platforms, and clinical winners.
Components such as chips, sensors, and energy harvesters receive investment in the early hardware stage because these components can be used to create a host of other
Platform plays include stable update infrastructure, model management for “regulated” devices, and these appeal to VCs interested in growth-stage investments with high margins.
Condition-specific device startups (hearing, cardiac, neuromodulation) present an area of interest for device
A market analysis in 2025 finds a scaling of investment and a desire for consolidation, with existing players acquiring skilled teams instead of rebuilding capabilities in-house. Startups need to be ready from a regulatory and evidence standpoint to generate the highest potential exit value.
Practical Advice For Patients, Practitioners, Startups
- Patients: Enquire about the type of data stored in the implant, what is uploaded, who approves the updates, and the lifespan of the device before being replaced.
- Clinicians: Demand access to transparent performance data, audit trails, and means to override or shape device behavior in the clinical setting. Early adopters can work with research teams to produce post-market data.
- Startups: design with the most draconian regulatory requirements you will be subject to, and invest in robust update paths, explainable models, and interfaces centered on humans. Consider clinical trials designed to establish clinical effectiveness in addition to the accuracy of disease detection.
Risks And Failure Modes: What To Watch For
- Model Drift. There is a physiological change, so an originally valid classifier will degrade unless monitored with change control procedures defined ahead of time.
- Security Breaches. Backdoor updates present an attack vector. Digitally signed updates with hardware-rooted keys mitigate risks.
- Overautomation. Machines functioning independently can pose unforeseen risks. Engineers must factor in a degree of control by a human operator.
Timeline
Realistic Rollout Timeline
There is a simulated adoptational route because
- Present 2 Years: Niche, high-end devices (cochlear, sophisticated cardiac monitoring) and closely monitored clinical use cases. Vendors begin with firmware updates and post-market data collection.
- 25 Years: Expanded use cases due to standardization and regulatory frameworks, maturation of energy-harvesting prototypes, and scaling of services offered by platforms for secure updates.
- 5+ Years: Mainstream implant devices for chronic care, warranting adaptation over decades, plus a market for related software services pending robust evidence and a public-trust sine qua non.
Final Takeaway: What Success Looks Like
Success will come when devices can be truly shown to enhance the lives of patients tangibly, with minimal added complexity and risks. This is a very skilled area of engineering, with a strong need for ethics and a clinical trials process with regulators who understand a continuous improvement approach.
Implant intelligence will enable quicker decision times and a reduced need for follow-up care within a medical setting. It won’t be an easy journey, but the progress being made with components, regulatory support, and clinical success means the revolution is brewing and one worth paying attention to.
Frequently Asked Questions
- Q: Are these implants currently safe?
A: Some initial commercial products and pilots look promising, although device class and evidence will be key to ensuring safety. Long-term data is required for implantable devices. - Q: Will my private health information be transmitted to the cloud?
A: Not necessarily so. Edge devices handle most communications internally and will only transmit summaries or notifications unless otherwise set up. This helps in preventing exposure of data. - Q: How long will these implants last?
A: Lifespan is a function of power design and battery management. Self-powered concepts and ultra-low power inference will drive lifespans into the years and decades. - Q: Who is in control of the updates in A:
A: Usually, the manufacturer and clinical staff handle updates, so a good system will have crypto-signature verification and audit logs to protect the patient. - Q: Which conditions will be most improved soon?
A: Hearing loss, heart rhythm disorders, epilepsy, and closed-loop neuromodulation represent an initial target area because these conditions involve a measurable signal with a clear action for improvement. - Q: How will doctors access the implant data?
A: Devices will be able to communicate summaries and event reports into clinical systems via standard interfaces, and with secure communication being addressed by regulators and industry, this is imminent.

